Engineering cell mechanics for targeted drug delivery
– Predictive modeling of nanoparticle–cell interactions
Controlling the physicochemical properties of nanoparticles is critical to the efficacy and safety of various biomedical applications. Despite the small size and low concentration of nanoparticles, their properties significantly change the interaction outcome with a human cell. Yet finding the optimal design parameters often relies on empirical correlations gained from brute force experiments, leaving a large parameter space underexplored with great potential for improvement. We are building predictive models that can be utilized to accelerate nanoparticle design based on various cell properties and the desired interaction outcomes. Further, we elucidate several biophysical insights about particle-induced cell membrane deformation that are key to the model accuracy. We aim to validate our model by comparing with various published experimental data and seeking new collaborations.
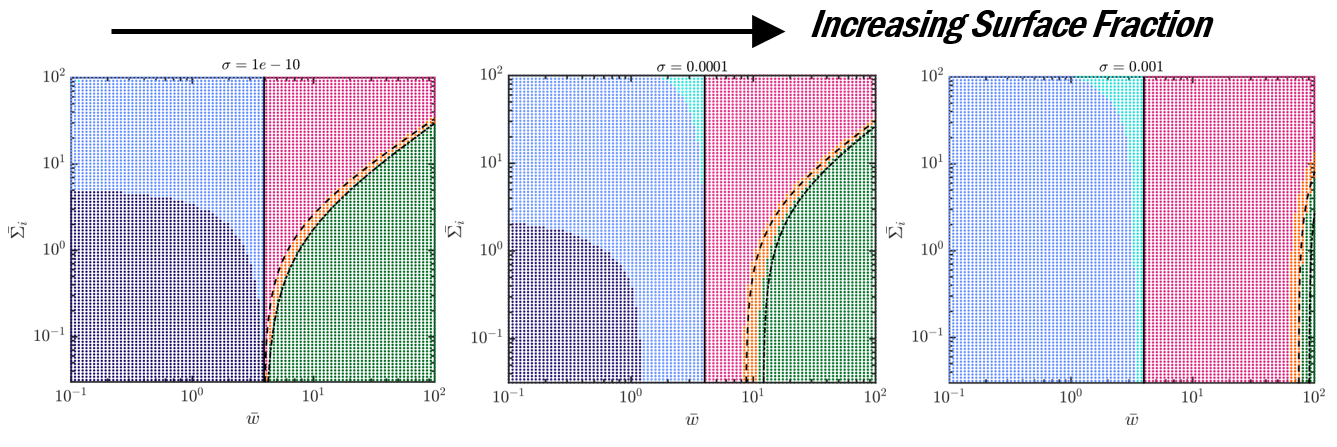
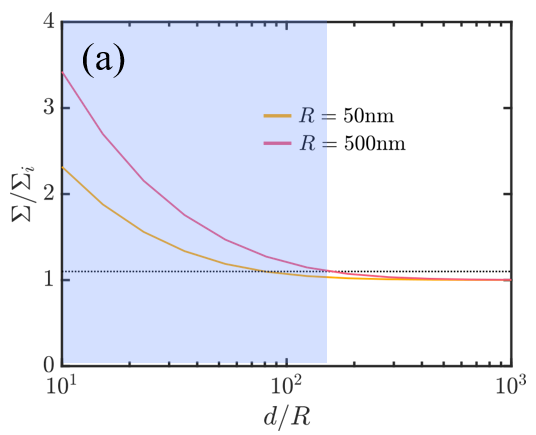
We (a) generate phase diagrams where each dot corresponds to one experiment and the color denotes the predicted particle-cell interaction outcomes and (b) perform scaling analysis to demonstrate the importance of particle-induced membrane tension changes for surface concentrations as low as 0.01%!
 |
 |
– Mechanics of particle-decorated therapeutic cells
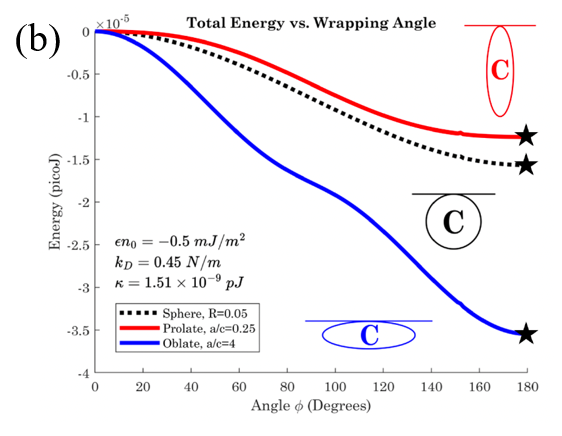
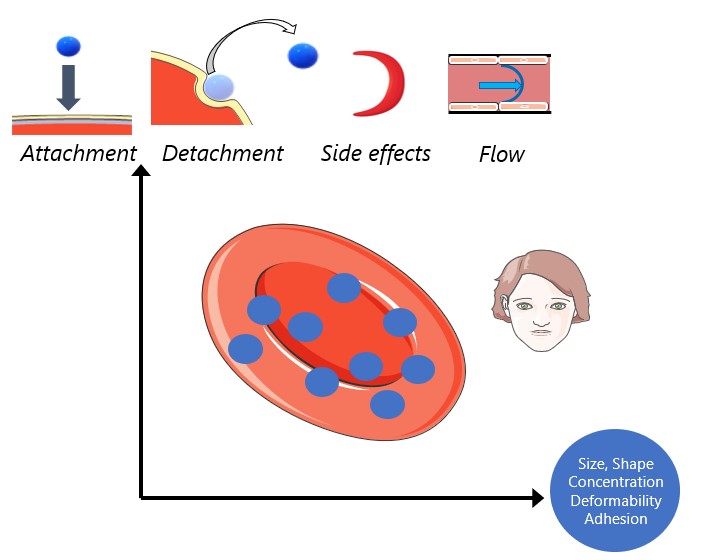
Cell therapy represents the future therapeutic landscape for treating diseases including cancer, infections and autoimmune disorders. While the interplay between mechanics and biology has been widely studied and emphasized in healthy and disease cells, its influence on engineered cells is often overlooked in the development of cell therapy. For example, when cell surface is decorated with drug-encapsulating particles, many open questions remain about how particle parameters influence cell mechanics and drug targeting efficiency. The natural targeting mechanism of blood cells (red blood cells, platelets, T cells, monocytes, natural killer cells etc.) deforming and traveling via microcirculation from the site of injection to the site of action is influenced by particles to an unknown extent, leading to concerns of side effects as well as engineering opportunities for controlling drug biodistribution. Our aim is to develop theoretical models of engineered cell mechanics based on particle-membrane interactions to guide drug carrier design. In recent work, we have shown that cell membrane tension induced by nanoparticle attachment can significantly alter particle uptake by engineered cells. This increased tension can block endocytosis and induce changes in particle adsorption isotherms and shear-induced particle detachment as in blood vessels. This platform technology has significant translational effects because the design of ideal drug carriers involves a large parameter space, and its optimization cannot be efficiently translated from animal models to human.
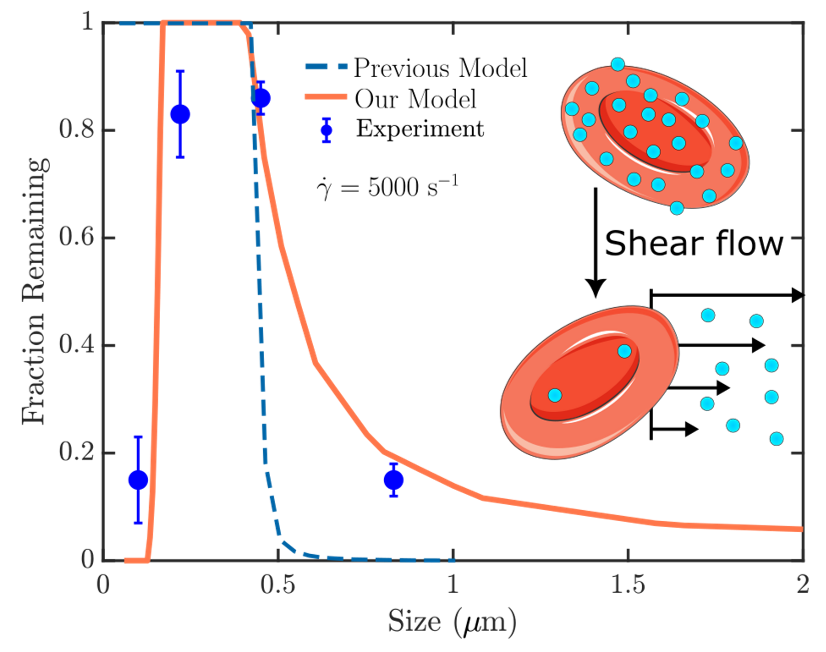
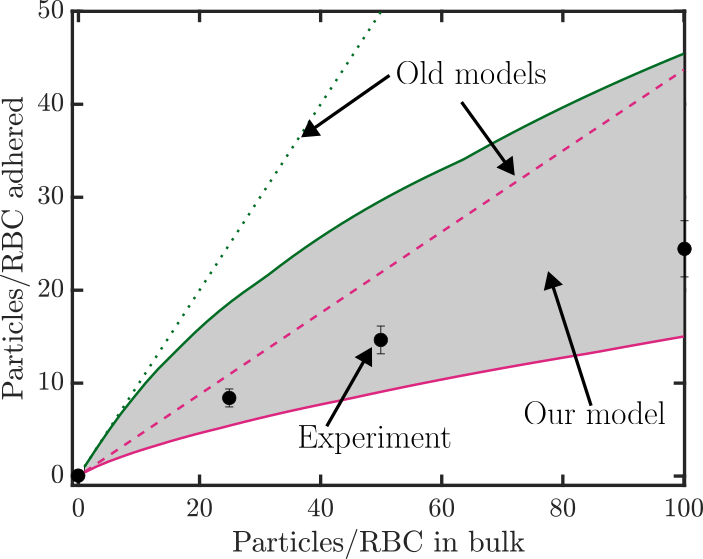
1Chambers, E., & Mitragotri, S. (2007). Experimental Biology and Medicine
2Anselmo, A. C., et al. (2013). ACS Nano
Collaborators:
Prof. Katherine Elvira, University of Victoria
Funding Sources:

NSF CAREER
MISTI Travel Award
23-24 Mathworks Fellowship
MIT FY 23 Research Support Committee Award
Awards:
XIXth International Congress of Rheology Jim Swan Graduate Student Poster Award