Multiscale Coarse-Grained Modeling of Blood Suspension Mechanics in Microchannel Flows
Human blood contains various types of circulating blood cells which differ in rigidity, shape, size, concentration etc., and result in distinct hydrodynamic mechanisms in small blood vessels influencing their spatial distributions. Such suspension mechanics occurring in microchannel flows have profound influences on blood pathophysiology, including platelet adhesion which can trigger hemostasis (stopping bleeding) or thrombosis (abnormal blood clotting). Developing a multiscale model relating molecular binding kinetics to macroscopic platelet adhesion dynamics in the presence of blood flow microhydrodynamics can significantly benefit the precise diagnosis and personalized treatment of various platelet-related disorders. However, existing large-scale simulations are not capable of predicting personal platelet adhesion dynamics at high throughput. We developed a coarse-grained model to decompose complex hydrodynamics in blood suspensions into a deformability-induced lift and two-cell interactions. Both mechanisms can be computed using multiple small-scale simulations running in parallel and are coupled with a multiscale biophysical modeling of platelet adhesion. This method reduces the simulation duration from multiple weeks to a single day and inspired multiple top-down and bottom-up studies of platelet disorders related to Bernard-Soulier syndrome, anemia, blood types, etc. This work was supported by Stanford Graduate Fellowship and was performed in collaboration with the Royal College of Surgeons in Ireland. A microfluidic diagnostic device based on this study is under development.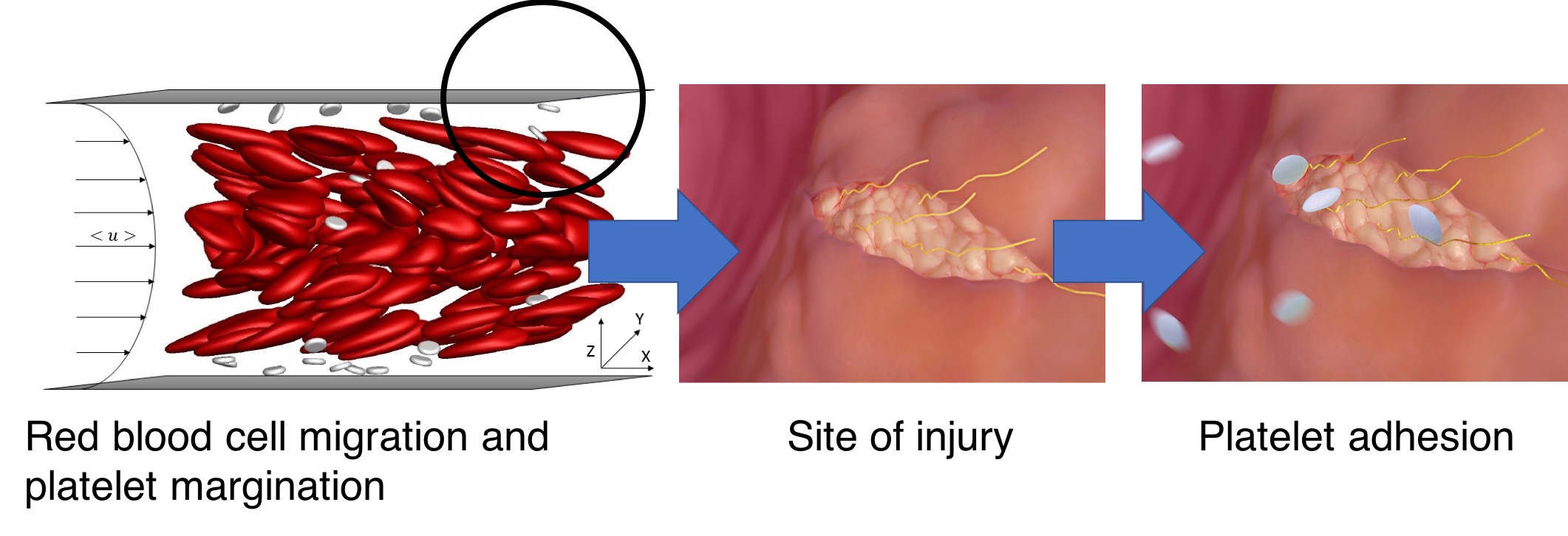
Subcutaneous Chip for Pharmacokinetic Testing
Subcutaneous injections are one of the most common routes of medication administration: they offer higher patient adherence and the benefit of self-administration compared to intravenous injection. However, estimating the pharmacokinetics of subcutaneously administered drugs is challenging in preclinical research primarily due to a lack of reliable in vivo and in vitro models. We developed a microfluidic subcutaneous model mimicking the diffusion and physiology of the subcutaneous environment for drug testing, utilizing multiscale principles of mass transfer and microhydrodynamics. This advanced in vitro model incorporates critical cellular elements in a controlled manner, thus overcoming limitations in the conventional in vivo animal models (e.g. injection site-to-site variations and variations across species) and acellular in vitro models (e.g. over-simplified geometry and a lack of cellular activities). Our technology will facilitate the development of subcutaneously administered drug formulations, especially biologics which are attracting immense research interests. This work was supported by Sanofi.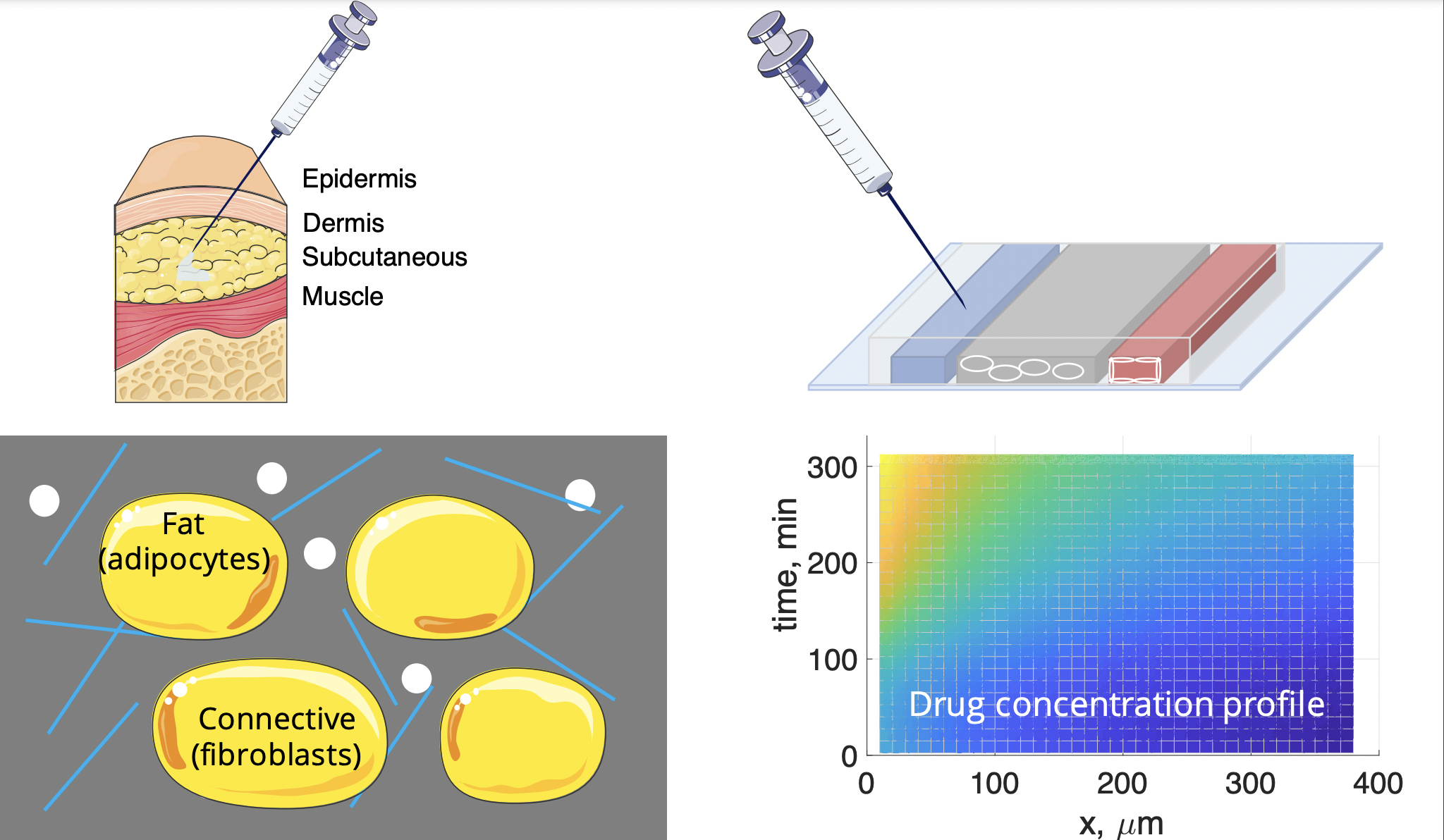
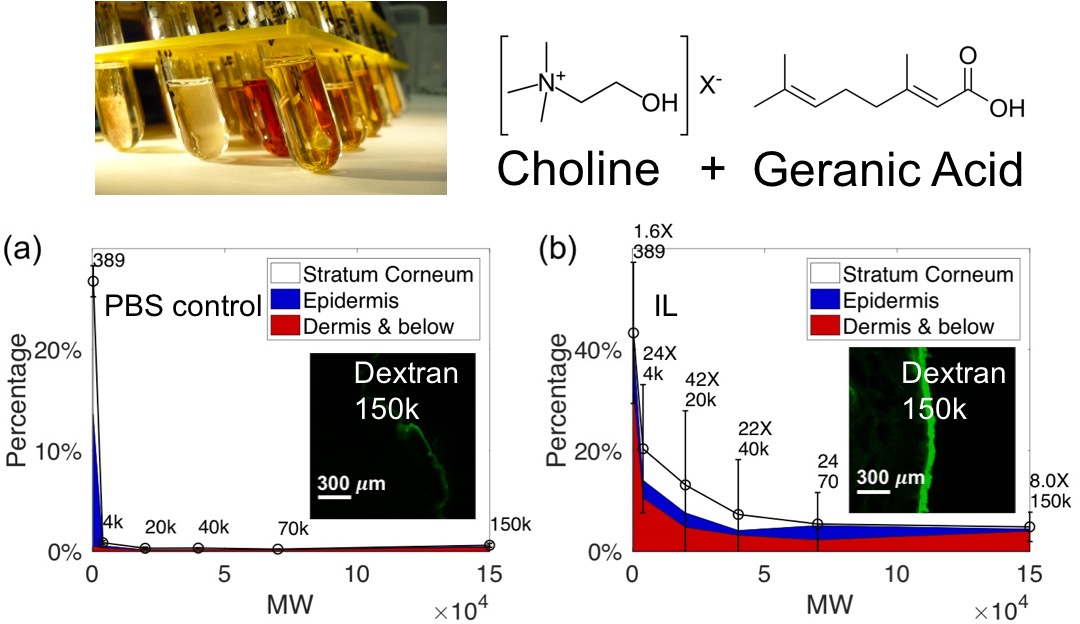
Transdermal transport of macromolecules enhanced by ionic liquids
Transdermal delivery of large hydrophilic molecules is a long-standing challenge owing to the strong diffusive barrier properties of the skin. Room-temperature ionic liquids (IL), i.e. low-melting-point liquid salts containing organic cations, are a novel class of chemical permeation enhancers with advantages such as low volatility, high recyclability, easy synthesis, and high tunability. A fundamental understanding of the interaction between ILs and the skin (e.g. lipids and proteins) enables more efficient exploration of the vast parameter space of ILs. IL-skin interactions differ from (1) conventional molecular chemical enhancers and (2) other permeation enhancement techniques such as electroporation and sonoporation. We combined ex vivo transport experiments, spectroscopic studies, and theoretical modeling to elucidate the novel properties of ILs. Our findings provide design principles of ILs to expand the scope of transdermal drug delivery as a noninvasive administration route.